Novici Consensus Interferon α:
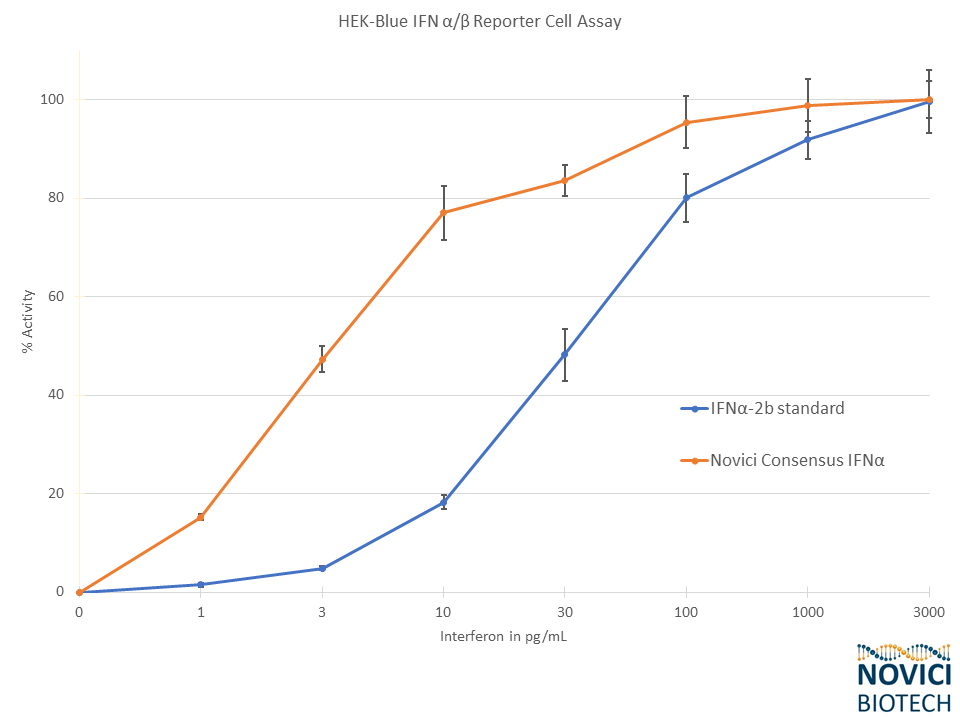
- Highly Active: Markedly higher specific activity than IFNα-2b in cellular bioassay
- Correct Size: Exact expected molecular weight confirmed by mass spectrometry
- Efficient Process: Our product is made in higher eukaryotic cells and does not require protein re-folding
- Safety: Infergen was already an FDA-approved drug for the treatment of chronic hepatitis C. If proven safe and effective for antiviral use, our version of this molecule could permit substantially lower dosing for respiratory viruses
Immunomodulators, such as recombinant human interferons (IFNs), are known to be broadly effective in the treatment of various human viruses. While there is still no agreement on the efficacy of interferon treatment in COVID-19, numerous clinical trials are underway to test their effectiveness. In China, interferon-α (IFNα) is recommended for the treatment of COVID-19. An initial retrospective study on Chinese patients indicates that IFNα can have a positive impact on disease outcome when administered early in the infection (Zhou 2020). Currently the United States recommends use in clinical trials only.
We successfully produced an array of engineered type I interferons with broadly-enhanced antiviral activities in the past with US military collaborators at USAMRIID (Koehler 2011). To address a potential need for recombinant IFNs in the fight against COVID-19, we have re-initiated and optimized production of a highly active synthetic human IFNα called Consensus IFNα (Ozes 1992) in plants. Consensus IFNα, also known as Interferon Alfacon-1, or by its trade name Infergen, is a highly active FDA-approved drug for the treatment of chronic hepatitis C virus. However, the manufacture and use of this effective drug was abandoned years ago in favor of small molecule antivirals. Novici’s plant-made Consensus IFNα has been tested in cellular bioassays and demonstrates characteristically high activity when compared to the IFNα-2b control. We believe that our plant-made version of Consensus IFNα might represent an effective and affordable route to prophylaxis and early treatment of COVID-19.
Click here to download an information sheet on our current lot of Consensus IFNα.
To request a free sample of Consensus IFNα for research use please email info@novicibiotech.com.
- Koehler JW, Dupuy LC, Garrison AR, Beitzel BF, Richards MJ, Ripoll DR, Wallqvist A, Teh S-Y, Vaewhongs AA, Vojdani FS, Padgett HS, Schmaljohn CS. Novel plant-derived recombinant human interferons with broad spectrum antiviral activity. Antiviral Research 92(3):461-9 (2011)
- Ozes ON, Reiter Z, Klein S, Blatt LM, Taylor MW. A comparison of interferon-Con1 with natural recombinant interferons-alpha: antiviral, antiproliferative, and natural killer-inducing activities. Journal of Interferon Research 12(1):55-9 (1992)
- Zhou Q, Chen V, Shannon CP, Wei1 X-S, Xiang X, Wang X, Wang Z-H, Tebbutt SJ, Kollmann TR, Fish EN. Interferon-α2b Treatment for COVID-19. Frontiers in Immunology 11:1061 (2020)

