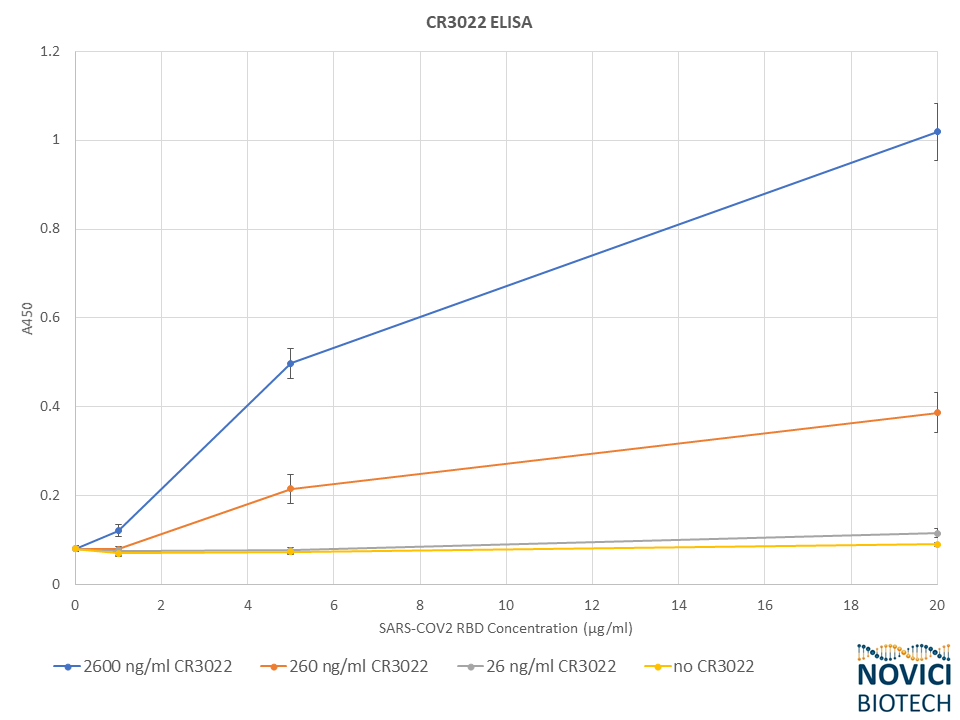
Early in 2020 we saw an opportunity to help address the emerging coronavirus threat by utilizing our skills in rapidly producing high-quality recombinant antibodies. With early evidence in the scientific literature that the monoclonal antibody CR3022 binds SARS-CoV-2 and no public source of any SARS-CoV-2 antibodies yet available, we decided in late January to produce it in our expression system as a vital reagent for coronavirus researchers. In under four weeks we designed, expressed, and purified recombinant human IgG1 and IgG4 CR3022 variants from plants. Shortly thereafter, we demonstrated binding of this antibody to recombinant SARS-CoV-2 viral glycoproteins, which we also produced ourselves using plants.
To ensure the broadest and most efficient distribution of this important antibody to the research community, in March 2020 we donated one of our first batches to BEI Resources, which distributes for shipping costs only (BEI catalog# NR-52392, register with BEI as BSL1 or higher to order). Due to high demand, we have subsequently re-supplied CR3022 under two different part numbers (BEI catalog# NR-53876 and NR-52392). Novici-produced CR3022 distributed by BEI Resources has already been cited in several publications including those listed below:
- Melani RD, Des Soye BJ, Kafader JO, Forte E, Hollas M, Blagojevic V, Negrão F, McGee JP, Drown B, Lloyd-Jones C, Seckler HS, Camarillo JM, Compton PD, LeDuc RD, Early B, Fellers RT, Cho B-K, Mattamana BB, Goo YA, Thomas PM, Ash MK, Bhimalli PP, Al-Harthi L, Sha BE, Schneider JR, Kelleher NL. Next-Generation Serology by Mass Spectrometry: Readout of the SARS-CoV-2 Antibody Repertoire. Journal of Proteome Research 21(1): 274-288 (2022) PMID: 34878788
- Garrett ME, Galloway J, Chu HY, Itell HL, Stoddard CI, Wolf CR, Logue JK, McDonald D, Matsen IV FA, Overbaugh J. High-resolution profiling of pathways of escape for SARS-CoV-2 spike-binding antibodies. Cell 184: 2927-2938 (2021) PMID: 34010620
- Puthenveetil R, Lun CM, Murphy RE, Healy LB, Vilmen G, Christenson ET, Freed EO, Banerjee A. S-acylation of SARS-CoV-2 spike protein: Mechanistic dissection, in vitro reconstitution and role in viral infectivity. J Biol Chem 297(4):101112 (2021) PMID: 34428449
- Shrivastava T, Singh B, Rizvi ZA, Verma R, Goswami S, Vishwakarma P, Jakhar K, Sonar S, Mani S, Bhattacharyya S, Awasthi A, Surjit M. Comparative Immunomodulatory Evaluation of the Receptor Binding Domain of the SARS-CoV-2 Spike Protein; a Potential Vaccine Candidate Which Imparts Potent Humoral and Th1 Type Immune Response in a Mouse Model. Frontiers in Immunology 12: 641447 (2021) PMID: 34108961
- Chellamuthu P, Angel AN, MacMullan MA, Denny N Mades A, Santacruz M, Lopez R, Bagos C, Casian JG, Trettner K, Lopez L, Nirema N, Brobeck M, Kojima N, Klausner JD, Turner F, Slepnev V, Ibrayeva A. SARS-CoV-2 Specific IgG Antibodies Persist Over a 12-Month Period in Oral Mucosal Fluid Collected From Previously Infected Individuals. Frontiers in Immunology 12:777858 (2021) PMID: 34956206
- Thomas A, Messer WB, Hansel DE, Streblow DN, Kazmierczak SC, Lyski ZL, Lu Z, Slifka MK. Establishment of Monoclonal Antibody Standards for Quantitative Serological Diagnosis of SARS-CoV-2 in Low-Incidence Settings. Open Forum Infectious Diseases 8:ofab061 (2021) PMID: 33723513
- Varghese FS, van Woudenbergh E, Overheul GJ, Eleveld MJ, Kurver L, van Heerbeek N, van Laarhoven A, Miesen P, den Hartog G, de Jonge MI, van Rij RP. Berberine and Obatoclax Inhibit SARS-Cov-2 Replication in Primary Human Nasal Epithelial Cells In Vitro. Viruses. 13: 282 (2021) PMID: 33670363
- Tsai W-Y, Ching LL, Hsieh S-C, Melish ME, Nerurkar VR, Wang W-K. A real-time and high-throughput neutralization test based on SARS-CoV-2 pseudovirus containing monomeric infrared fluorescent protein as reporter. Emerging Microbes & Infections 10(1): 894-904 (2021) PMID: 33929934
- Bates TA, Weinstein JB, Farley S, Leier HC, Messer WB, Tafesse FG. Cross-reactivity of SARS-CoV structural protein antibodies against SARS-CoV-2. Cell Rep. 34:108737 (2021) PMID: 33545052
- Zeng C, Evans JP, Pearson R, Qu P, Zheng Y-M, Robinson RT, Hall-Stoodley L, Yount J, Pannu S, Mallampalli RK, Saif L, Oltz E, Lozanski G, Liu S-L. Neutralizing antibody against SARS-CoV-2 spike in COVID-19 patients, health care workers, and convalescent plasma donors. JCI Insight 5:e143213 (2020) PMID: 33035201
- Itell HL, Weight H, Fish CS, Logue JK, Franko N, Wolf CR, McCulloch DJ, Galloway J, Matsen IV FA, Chu HY. SARS-CoV-2 Antibody Binding and Neutralization in Dried Blood Spot Eluates and Paired Plasma. Microbiology Spectrum 9(2):e0129821 (2021) PMID: 34668728
- Kelsen SG, Braverman AS, Patel P, Aksoy MO, Hayman J, Rajput C, Ruggieri Sr. MR, Gentile N. Heightened COVID-19 Vaccine Response Following SARS-CoV-2 Infection. medRxiv 2021.03.18.21253845; doi: https://doi.org/10.1101/2021.03.18.21253845
- Selma-Royo M, Bauerl C, Mena-Tudela D, Aguilar-Camprubi L, Perez-Cano FJ, Parra-Llorca A, Lerin C, Martinez-Costa C, Collado MC. Anti-Sars-Cov-2 IgA and IgG in Human Milk After Vaccination is Dependent on Vaccine Type and Previous Sars-Cov-2 Exposure: A Longitudinal Study. medRxiv 2021.05.20.21257512v1 doi: https://doi.org/10.1101/2021.05.20.21257512
- Kelsen SG, Braverman AS, Aksoy MO, Hayman JA, Patel P, Rajput C, Zhao H, Fisher SG, Ruggieri MR, Gentile NT. A Longitudinal Study of BNT162b2 Vaccine-Induced Humoral Response and Reactogenicity in Health Care Workers with Prior COVID-19 Disease. medRxiv 2021.03.18.21253845v2 doi: https://doi.org/10.1101/2021.03.18.21253845doi
- Minami SA, Jung S, Huang Y Harris BS, Kenaston MW, Faller R, Nandi S, McDonald KA, Shah PS. Production of novel Spike truncations in Chinese hamster ovary cells. bioRxiv 2021.12.06.471489v1 doi: https://doi.org/10.1101/2021.12.06.471489
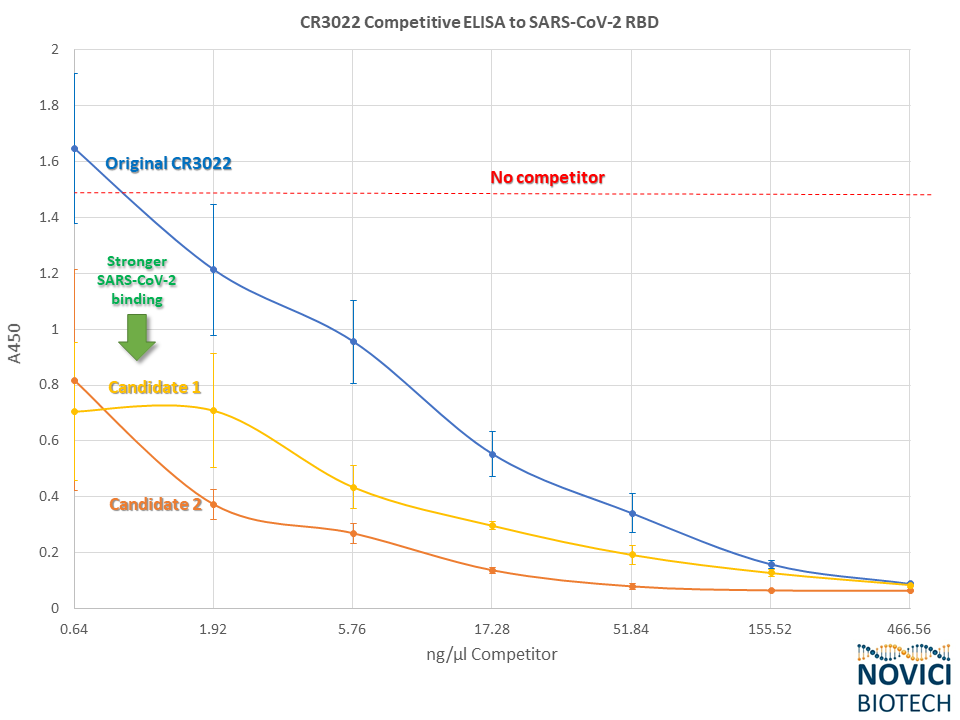
We have continued to develop CR3022 by engineering bispecifics and screening libraries of variants with significantly improved binding characteristics over the original antibody. Two exemplary candidates with improved binding to SARS-CoV-2 are shown below in a competitive ELISA.